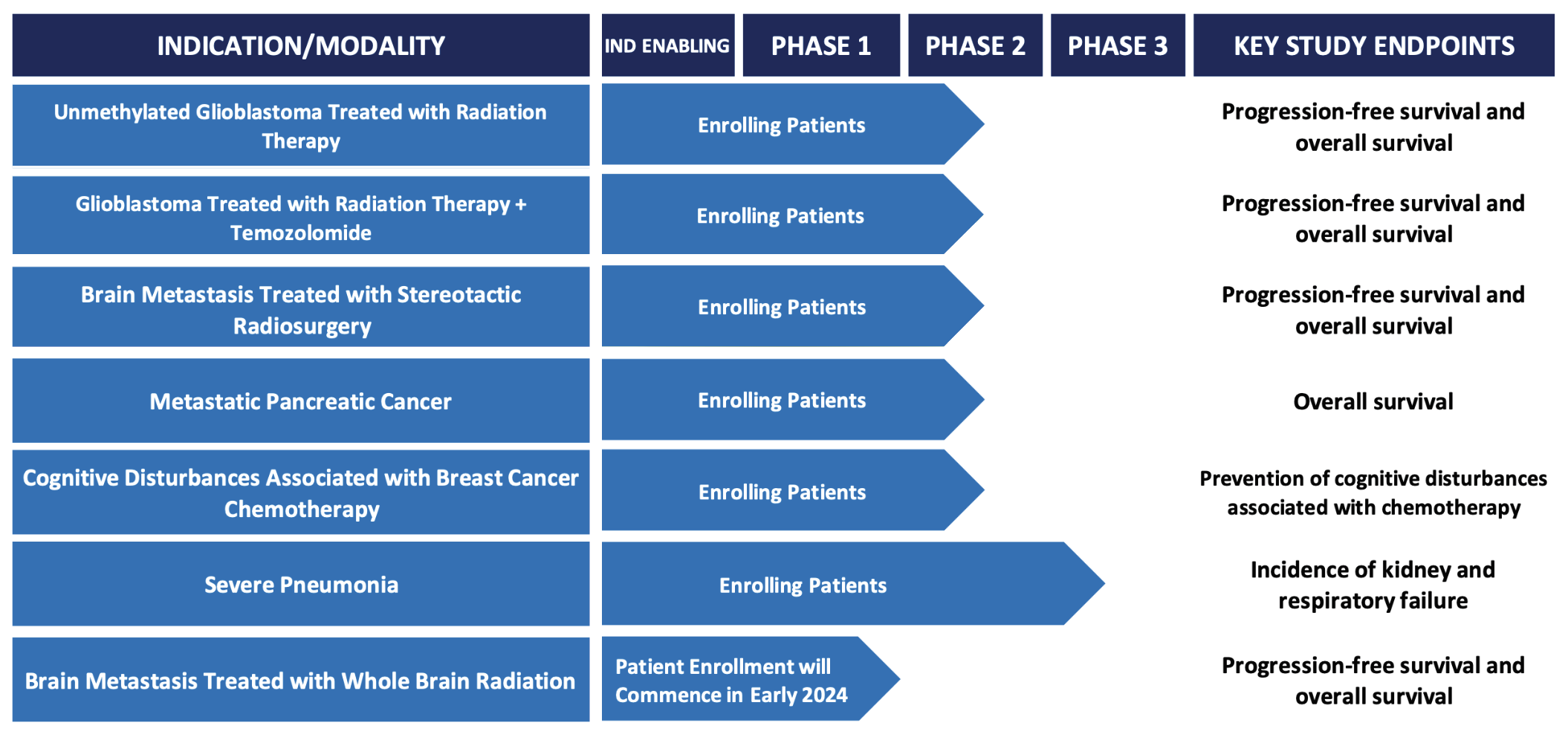
Cantex is developing Azeliragon for the treatment of glioblastoma, brain metastases, pancreatic cancer, breast cancer, and prevention of acute kidney injury in hospitalized pneumonia patients.
- Azeliragon, is an oral, small molecule, Phase 2-ready compound, administered once-daily, that inhibits RAGE interactions with its natural ligands, including HMGB1 and S100 proteins, in the tumor microenvironment. Activation of RAGE by these ligands stimulates cancer and its progression and metastasis and resistance to cancer treatment.
- Azeliragon was licensed in mid 2021 from vTv Therapeutics Inc. (NASDAQ:VTVT) and was originally in development by vTv for Alzheimer’s disease. Clinical safety data, involving over 2000 patients treated for up to 18 months, indicated a high level of safety and tolerability of azeliragon.
- There has been extensive demonstration of pre-clinical efficacy of RAGE inhibition in animal models of several cancers, including pancreatic cancer, glioblastoma, and brain metastasis from breast and lung cancer, as well as diverse serious complications of cancer, including cancer-related cognitive decline, as well as the development of metastatic disease.
- Azeliragon is in clinical development for the treatment of glioblastoma, brain metastasis, pancreatic cancer, breast cancerand prevention of acute kidney injury in hospitalized pneumonia patients. Azeliragon has several issued patents including composition of matter patents that protect azeliragon from generic competition until at least May 2034.
- Azeliragon also has orphan drug designation for the treatment of glioblastoma, conferring seven years of protection for that indication from generic competition.